Marcin is a master student from Warsaw University of Technology. He will focus on enantioselective epoxidation of enones. Good luck Marcin!
PostDoc Aleksandra Siklitckaia joined the group!
Aleksandra will work on homogeneous catalysis problems, especially on non-linear effects. She will also contribute to our research in heterogeneous catalysis. Good luck Sasha!
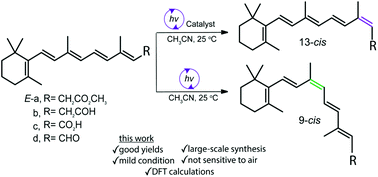
Further on retinoids isomerization: this time light!
Happy to announce new paper from our group:
Z-isomerization of retinoids through combination of monochromatic photoisomerization and metal catalysis
Catalytic Z-isomerization of retinoids to their thermodynamically less stable Z-isomer remains a challenge. In this report, we present a photochemical approach for the catalytic Z-isomerization of retinoids using monochromatic wavelength UV irradiation treatment. We have developed a straightforward approach for the synthesis of Z-retinoids in high yield, overcoming common obstacles normally associated with their synthesis. Calculations based on density functional theory (DFT) have allowed us to correlate the experimentally observed Z-isomer distribution of retinoids with the energies of chemically important intermediates, which include ground- and excited-state potential energy surfaces. We also demonstrate the application of the current method by synthesizing gram-scale quantities of 9-cis-retinyl acetate 9Z-a. Operational simplicity and gram-scale ability make this chemistry a very practical solution to the problem of Z-isomer retinoid synthesis.
Read more: https://pubs.rsc.org/en/content/articlelanding/2019/ob/c9ob01645g
Openings in CoopCat project!
We are hiring! We look for a skilled PhD student and post-doctoral researcher to join CoopCat team. Student positions are available as well. See details in Jobs section!
Paper about E/Z isomerization of retinoids accepted in Dalton Trans.
Our work on catalytic E/Z isomerization of retinoids was just accepted in Dalton Transactions! It is already online as ‘just accepted’ unformated paper:
https://pubs.rsc.org/en/content/articlelanding/2019/dt/c9dt02189b#!divAbstract
This work is a result of our collaboration with Prof. Palczewski group at UC Irvine.
Our works highlighted by EurekAlert!
Happy to share press releases about our two recent papers:
Hidden gapless states on the path to semiconductor nanocrystals about our Mater. Horiz. paper.
The road to green hydrogen runs through mazes in algal proteins about our Nat. Chem. contribution!
Interview with Adam Kubas in TOK FM radio
 Adam was a guest of Cezary Łasiczka in TOK FM radio. He talked about perspectives of hydrogen production and its use as a fuel.
Adam was a guest of Cezary Łasiczka in TOK FM radio. He talked about perspectives of hydrogen production and its use as a fuel.
You can listen the podcast HERE (only in Polish sorry).
Hello!
Welcome to the website of the CoopCat project group led by Adam Kubas in the Institute of Physical Chemistry. We are part of the Modern Heterogeneous Catalysis Group (MoHCa).